The assembly of the embryonic cornea from ectoderm an neural crest cells is precisely orchestrated by integration of multiple cellular events, including migration, induction, and differentiation. Neural crest cells (NCCs) arising in the neural tube migrate toward the developing optic cup under control of various attractive and repellent molecules. Upon arrival in the periocular region, NCCs appear to follow different developmental programs which result in determination toward corneal stromal, corneal endothelium, or other periocular tissue fates.
In avian models, NCCs arrive in the periocular region but are prevented from entering the presumptive cornea by close apposition of the optic cup to the overlying ectoderm. Later this physical obstacle is removed as the optic cup pulls away from the ectoderm, but NCCs are still prevented from entering the cornea by inhibitory signaling semaphorin 3A (Sema3A), a lens-derived factor, acting on neuropilin 1 (Nrp1) which is expressed in the NCCs. Eventually, some NCCs down-regulate Nrp1, and are thus able to migrate into the presumptive cornea. They form a single cell layer and appear to give rise to the corneal endothelium. Later, more NCC-derived cells in the periocular region migrate between the endothelium and ectoderm to give rise to the stroma.
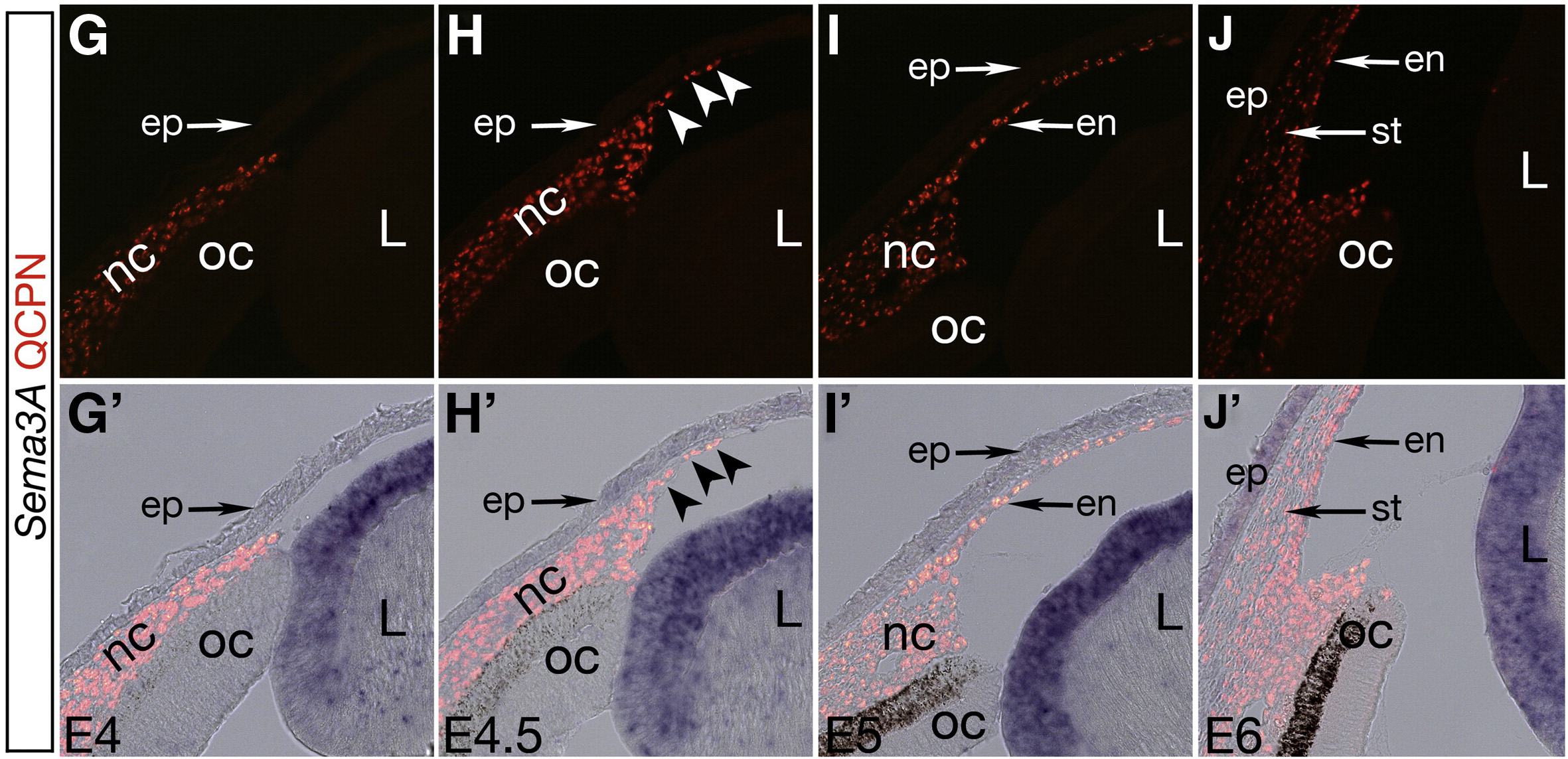
In the mouse, this process unfolds quite differently in that NCCs migrate directly into the presumptive cornea without first pausing in the periocular space. Differentiation of cell layers appears to occur after all cells are present in the cornea, rather than during corneal invasion.
It is clear that different species use different mechanisms to establish different cells and tissue fates in the cornea. We hypothesize that in some cases, NCCs begin to establish fate based on their position during migration, and so this position helps to direct them toward a differentiated fate. We are using several techniques including retrovirus labeling, photoconversion, and 4-dimensional live imaging to identify the earliest phases of NCC migration at which corneal fate can be predicted.
At present it is unclear to what extent NCC migration and corneal invasion is controlled by programs innate to the NCCs, versus induced by their microenvironment. To shed further light on this matter we are investigating observed species differences in NCC corneal invasion. We are also interested in the mechanisms that regulate NCC homing to the periocular region and invasion of the presumptive cornea, which appear to have some important differences as compared to other NCC streams. To this end, we are examining retiniod control of these processes in the early embryo.